Published date: Aug 26, 2018 Tags: 5328-37-0 Plant derived arabinose L-arabinose L-Arabinopyranose plant source NAD plant origin China GMP Factory
What is L-Arabinose
L-Arabinose is a naturally occurring pentose with a sweet taste and is widely distributed as a component of complex non-starch polysaccharides in plant cell walls and plant gums.
As a low-calorie sugar-substituting sweetener, the relative sweetness of L-Arabinose equals to half of the Sucrose’s. Some research suggested that L-Arabinose selectively inhibit sucrose absorption and then inhibit the increase of blood sugar.
Furthermore, L-Arabinose is an intermediate in flavor industry for producing flavors, the derivates of L-Arabinose can be used in the development of antiviral agents such as nucleoside.
Properties
| CAS No: | 5328-37-0 |
| EINECS: | 226-214-6 |
| Alias Name: | L-(+)-Arabinose; L-Arabinopyranose |
| Chemical Structure: | 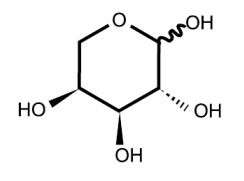 |
| Molecular Formula: | C5H10O5 |
| Molecular Weight: | 150.13 |
| Assay: | NLT 99.0% |
| Solubility: | Easily soluble in water, slight soluble in alcohol |
| Specific Rotation: | +101º to +105º |
| Application: |
Intermediate for nucleoside derivates synthesis
Intermediate for flavors synthesis Ingredient in function food
Food additive in foods, drinks
|
History
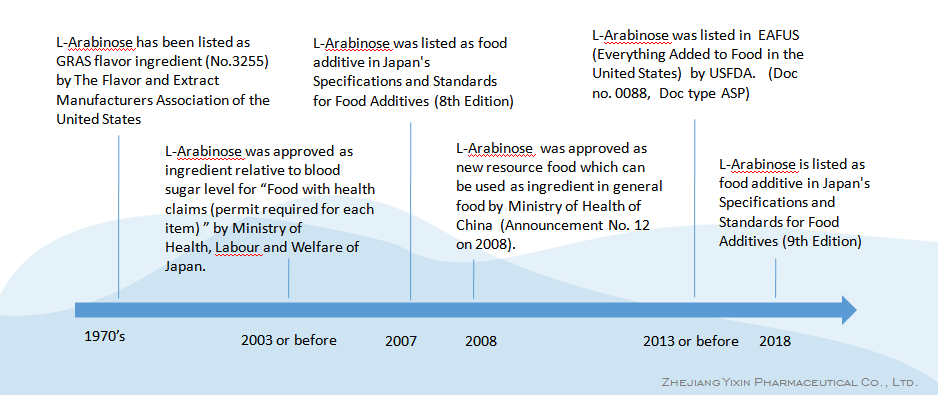
-1970's, L-Arabinose has been listed as GRAS flavor ingredient (No.3255) by The Flavor and Extract Manufacturers Association of the United States
-2003 or before, L-Arabinose was approved as ingredient relative to blood sugar level for "Food with health claims (permit required for each item)" by Ministry of Health, Labour and Welfare of Japan.
-2007, L-Arabinose was listed as food additive in Japan's Specifications and Standards for Food Additives (8th Edition)
-2008, L-Arabinose was approved as new resource food which can be used as ingredient in general food by Ministry of Health of China (Announcement No. 12 on 2008).
-2013 or before, L-Arabinose was listed in EAFUS (Everything Added to Food in the United States) by USFDA. (Doc no. 0088, Doc type ASP)
- 2018, L-Arabinose is listed as food additive in Japan's Specifications and Standards for Food Additives (9th Edition)
Benefits
Sugar-substituting sweetener
L-Arabinose has a nice, sweet taste that does not linger in the mouth and has no aftertaste. No-calorie sweeter, it's not metabolized in humans. No side-effects were reported
Inhibiting sucrose absorption
The sucrase enzyme normally splits the sucrose molecule into one D-glucose and one D-fructose molecule. During the period that the sucrase enzyme is inhibited by L-Arabinose, the splitting of sucrose is delayed or even prevented, and the release of D-glucose and D-fructose to the blood is delayed.
Therefore L-Arabinose selectively inhibits the sucrase enzyme in the small-intestine in an uncompetitive manner.
Dosage: add 3% -5% L-Arabinose in Sucrose.
Raw material for Drug Synthesis
L-Nucleosides and their analogues have become useful agents for the treatment of viral diseases due in part to their good antiviral activity and generally low toxicity.
Yixin’s advantages
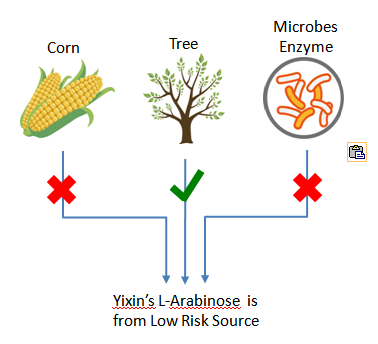
Plant-derived, 100% Non-GMO Source
Yixin’s plant derived L-Arabinose is completely from nature tree, non GMO source. It does NOT have any GMO risk, neither Yixin nor any of Yixin suppliers of raw materials has used Genetically Modified Organisms in this product.
 |
|
Clean Technology
Yixin plant derived L-arabinose is produced by clean technology, during the whole production process, only water is used as extraction solvent to ensure the environmental sustainability and the process safety.
Low Impurity Profile
Yixin plant derived L-arabinose is produced under GMP system, every batch is tested for its chemical impurities and chiral impurity to meet the quality requirements both in pharmaceutical and food industry.
GMP system
Yixin plant derived D-galactose is produced under GMP system to ensure the traceability and consistent supply. Yixin GMP system was established and improved over 20 years, for carbohydrates facility, NSF GMP certificate has been obtained since 2015, In 2015 Yixin carbohydrate facility was inspected and concluded by US FDA to meet the related requirements.
-In 2015-2018 Yixin Carbohydrates facility was assessed by NSF international and found to be in compliance with GMP Requirements in NSF/ANSI Standard173,Section B Dietary Supplements.
-Yixin Carbohydrates Facility was inspected in 2015 by US.FDA, it’s concluded to meet the related requirement of Dietary Supplement Ingredient.
-In 2015-2018 Yixin Carbohydrates facility were certificated by 3rd Kosher certification organization.
-Since 2002, Yixin has obtained the Certificates of GMP from CFDA for 9 dosage forms successively including capsules, tablets, syrup, preparation for external use and etc. In 2006 and 2011 Yixin facility of API was inspected and classified as acceptable by US FDA.
 EN
EN CN
CN DE
DE FR
FR JA
JA RU
RU